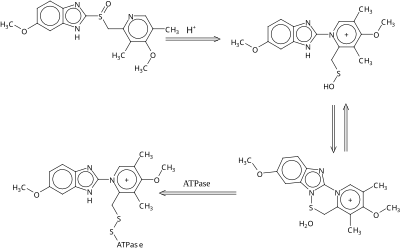
Omeprazole is a racemate. It contains a tricoordinated sulfur atom in a pyramidal structure and therefore can exist in equal amounts of both the S and R enantiomers. In the acidic conditions of the stomach, both are converted to achiral products, which reacts with a cysteine group in H+/K+ ATPase, thereby inhibiting the ability of the parietal cells to produce gastric acid.
Facing the loss of patent protection and competition from generic drug manufacturers, AstraZeneca developed and heavily marketed esomeprazole (Nexium) as a replacement in 2001. Esomeprazole is the S-enantiomer in the pure form.
omeprazole undergoes a chiral shift in vivo which converts the inactive R-enantiomer to the active S-enantiomer doubling the concentration of the active form. This chiral shift is accomplished by the CYP2C19 isozyme of cytochrome P450, which is not found equally in all human populations. Those who do not metabolize the drug effectively are called "poor metabolizers." The approximate proportion of the poor metabolizer phenotype in different populations is as follows:
Caucasians 10%
Asian 20%
South Pacific Islands 70%
In theory, by using pure esomeprazole the effect on the proton pump will be equal in all patients, eliminating the "poor metabolizer effect".



0 comments:
Post a Comment